You are here
Taxonomy
Heteropoda
EOL Text
Barcode of Life Data Systems (BOLD) Stats
| Specimen Records: | 31 | Public Records: | 18 |
| Specimens with Sequences: | 22 | Public Species: | 7 |
| Specimens with Barcodes: | 20 | Public BINs: | 9 |
| Species: | 7 | ||
| Species With Barcodes: | 7 | ||
Collection Sites: world map showing specimen collection locations for Heteropoda![]()
Heteropoda is a genus of spiders in the family Sparassidae, the huntsman spiders. They are mainly distributed in tropical Asia and Australia, while at least one species, H. venatoria, has a cosmopolitan distribution,[1] and H. variegata occurs in the Mediterranean.[2]
These spiders catch and eat insects, but in a laboratory study one species readily ate fish and tadpoles when offered,[1] and H. venatoria has been known to eat scorpions and bats.[3]
The largest species in the genus, and probably the whole family, is H. maxima, which is about 4.6 centimeters long but has a legspan of up to 30 centimeters.[4]
Species[edit source | edit]
As of 2006, there were about 180 to 190 species in the genus, but the group requires taxonomic study and revision.[5][6]
Species include:
- Heteropoda acuta Davies, 1994 — Queensland, Australia
- Heteropoda amphora Fox, 1936 — China, Hong Kong
- Heteropoda cervina L. Koch, 1875 — Queensland
- Heteropoda dagmarae Jäger & Vedel, 2005 — Laos, Thailand
- Heteropoda davidbowie Jäger, 2008 — Malaysia, Singapore, Sumatra
- Heteropoda fischeri Jäger, 2005 — India
- Heteropoda hosei Pocock, 1897 — Borneo
- Heteropoda maxima Jäger, 2001 — Laos
- Heteropoda venatoria Linnaeus, 1767 — Pantropical
- Heteropoda zuviele Jäger, 2008 — Vietnam
References[edit source | edit]
| Wikimedia Commons has media related to: Heteropoda |
- ^ a b Airamé, S. and P. Sierwald. (2000). Hunting and feeding behavior of one Heteropoda species in lowland rainforest on Borneo (Aranae, Sparassidae). Journal of Arachnology 28(2), 251-53.
- ^ Jager, P. and H. Ono. (2000). Sparassidae of Japan. I. New species of Olios, Heteropoda, and Sinopoda, with notes on some known species (Araneae: Sparassidae: Sparassinae and Heteropodinae). Acta Arachnologica 49(1), 41-60.
- ^ Ross, J., et al. (1982). The life cycle of Heteropoda venatoria (Linnaeus) (Araneae: Heteropodidae). Psyche 89, 297-306.
- ^ Iaeger, P. (2001). A new species of Heteropoda (Araneae, Sparassidae, Heteropodinae) from Laos, the largest huntsman spider? Zoosystema 23(3), 461-66.
- ^ Jäger, P. (2005). A 'swimming' Heteropoda species from Borneo (Araneae, Sparassidae, Heteropodinae). Journal of Arachnology 33(3), 715-18.
- ^ Eusemann, P. and P. Jäger. (2006). Heteropoda schwendingeri Jäger 2005 (Araneae: Sparassidae) – first description of female with notes on intraspecific variation and evidences supporting species status. Zootaxa 1325, 327-34.
| This spider-related article is a stub. You can help Wikipedia by expanding it. |
| License | http://creativecommons.org/licenses/by-sa/3.0/ |
| Rights holder/Author | Wikipedia |
| Source | http://en.wikipedia.org/w/index.php?title=Heteropoda&oldid=571537435 |
The heteropods are a group of pelagic snails (Class Gastropoda) that are found in moderate to low abundances, primarily in tropical to subtropical latitudes. Among the gastropods, they have four striking adaptations to the open ocean environment. First, the bodies and shells are largely transparent; only the buccal mass, eyes and viscera are opaque. Because of this transparency, one can view internal structures in the head region (see first photograph below). Second, the foot, which in bottom-dwelling snails is the sole-like structure used for crawling along substrates, is laterally-compressed to form a ventral swimming fin. Because of the weight of the dorsal visceral mass, heteropods swim "upside-down", with the fin directed upward. Third, they possess image-forming eyes, with a large, spherical lens and a basal, ribbon-like retina (see second photo below). And, fourth, the radula has elongate, sickle-shaped lateral and marginal teeth that are used to snare prey after the radula is protruded from the mouth, which is located at the tip of their mobile proboscis. The presence of a trunk-like proboscis is responsible for their common name, "sea elephants."
Diagnosis
Gastropod mollusks with:
- Bodies that are largely transparent
- Paired image-forming eyes with large, spherical lenses
- Laterally-flattened ventral swimming fin
- Radula with elongate, sickle-shaped teeth that are used to snare prey
| License | http://creativecommons.org/licenses/by/3.0/ |
| Rights holder/Author | Roger R. Seapy, Tree of Life web project |
| Source | http://tolweb.org/Pterotracheoidea/27801 |
All heteropods swim with their ventral side directed upward and the dorsal side, with the shell (when present) and visceral mass downward. Steady swimming is by undulations of the fin. In atlantids, a considerable side-to-side body motion is induced by the large fin, although the shell and keel act to partially offset the fin's movements. The large, elongate bodies of the carinariids and pterotracheids greatly dampen the side-to-side body motion. In the latter two groups, and especially in the pterotracheids, flexion of the trunk and tail are used in accelerated swimming during pursuit of prey or evasion of predators. The bodies of pterotracheids are the most elongate and streamlined of the three families, and they are the fastest swimmers.
The atlantids are negatively buoyant. During the day they must swim to maintain position. At night, however, they secrete strands of buoyant mucus, up to 0.5 m long, from which they are suspended (Lalli and Gilmer, 1989; Newman, 1990). Carinariids and pterotracheids have been observed to float motionless without sinking (Lalli and Gilmer, 1989). Their neutral buoyancy results from the large amounts of gelatinous tissue, mostly in the trunk and tail, that is made positively-buoyant by ion regulation; i.e., by replacing heavier sulfate ions by lighter chloride ions (up to 75% in Pterotrachea coronata) (Denton and Shaw, 1961).
Heteropods are carnivores that feed during the day, locating their prey visually. The eyes are unusual because they have a narrow, strip-like retina. Image formation is accomplished by scanning-eye movements. In atlantids, the only family studied to date, the retina is rotated through a 90° arc, from downward vertical to horizontal (Land, 1982). Presumably, prey are located beneath the animal by light reflected off the prey's body. In all heteropods, prey are captured by elongate, hooked radular teeth (described above). In the atlantids, the fin sucker is large and is used to hold the prey while pieces of tissue are torn off and ingested. In the carinariids and pterotracheids prey are ingested whole. The fin sucker is much smaller in these two groups and does not appear to be used in feeding. It is directed ventrally to posteroventrally and is spatially separated from the mouth; its primary or sole function is presumably to hold males and females together during mating.
Heteropods feed on a variety of zooplanktonic prey. Atlantids feed preferentially on other gastropods, especially shelled pteropods (Richter, 1968; Newman, 1990), and carinariids feed selectively on soft bodied prey (e.g., salps, doliolids and chaetognaths in Carinaria japonica; Seapy, 1980). Little is known of feeding in pterotracheids.
Defense against predators is based largely on transparency, although the buccal mass, pigmented region of the eyes, and the visceral mass (or visceral nucleus) are opaque. In Pterotrachea behavioral orientation and surface reflectivity of the pigmented portion of the eyes and the visceral nucleus reduce the visibility of these structures to upward-searching predators (Seapy and Young, 1986).
| License | http://creativecommons.org/licenses/by/3.0/ |
| Rights holder/Author | Roger R. Seapy, Tree of Life web project |
| Source | http://tolweb.org/Pterotracheoidea/27801 |
- Eyes
- Eyes image-forming, located dorsally in the head region at the anterior end of the trunk

Figure. Dorsal view of head region in Pterotrachea hippocampus. © Roger R. Seapy
- Eye structure complex, consisting of a distal, large spherical lens, an intermediate region with a pigmented external wall (with or without a clear dorsal window), and a basal ribbon-like retina
- Eye shape, viewed from dorsal aspect, generally triangular to rectangular
- Eye enclosed in gelatinous capsule (visible immediately distal to the lens in the photograph above)
- Swimming fin, sucker and operculum
- Foot laterally compressed as a swimming fin, which usually has a posterior to postero-ventral sucker (see title illustrations and photograph below)
- Fin sucker much larger in the Atlantidae than in the Carinariidae and Pterotracheidae (see title illustrations). In the atlantids it serves the function of holding prey while the radula tears off pieces of prey tissues for ingestion. The sucker is much smaller in carinarids and, even more so, in pterotracheids (where it occurs only in males). In both of the latter families the function of the sucker appears to be limited to holding males and females together during copulation, thus facilitating spermatophore transfer to the female
- Operculum present in all larval stages of heteropods, but it is only retained following metamorphosis in the adults of the family Atlantidae. In post-metamorphic atlantids the operculum is a flexible, thin cartilaginous structure that is born on an opercular lobe, located on the posterior margin of the swimming fin. The operculum serves to close off the shell aperture after animals retract into the shell (see the Atlantidae page)
- Proboscis and radula
- Proboscis muscular and mobile, extending anteriorly from the head and bearing a terminal buccal mass that contains the radula


Figure. Dorsal views of head and proboscis (left) and buccal mass with radula (right) of a female Pterotrachea scutata. © 2005 Roger R. Seapy
- Radula taenioglossate with a tooth formula of 2-1-R-1-2; i.e., each tooth row consists of two marginal teeth and one lateral tooth on either side of the central or rachidian tooth. Rachidian tooth morphology differs between the three families. Lateral and marginal teeth have elongated shafts and curved, hook-shaped ends


Figure. Dorsal views of atlantid radulae. Left: radula dissected from unidentified species of Atlanta. © 2005 Roger R. Seapy Right: scanning electron micrograph of Atlanta californiensis radula. Modified from Seapy and Richter (1993, Fig. 8b)
- Radular tooth shapes change during ontogenesis, with the result that position on the radula of the tooth row can change the appearance of the teeth (Richter, 1961, 1963). Morphogenetic changes in the teeth continue for a large part of radular growth in the Atlantidae, while in the other two families change is restricted to the late larval and early post-larval stages
- Two markedly different types of radulae are distinguished in the heteropods (Richter, 1961). In Type I radulae, the number of tooth rows increases continuously with growth, with the result that all stages of change in tooth shape are represented in mature animals and that teeth produced first on the radula are not cast off the anterior end of the radula after they are no longer of use in prey capture. In Type II radulae, teeth are cast off the anterior end of the radula once the radula has reached its specific number of tooth rows; thus the radula has a limited number of tooth rows. Type II radulae are seen in all Carinariidae and Pterotracheidae, while in the Atlantidae both types are seen (13 species have Type I and 8 species have Type II radulae)
- Shell
- A coiled shell is present in the larvae of all heteropods. Following metamorphosis, however, the larval shell is retained only in the Atlantidae and Carinariidae, where it forms the protoconch of the adult shell. In Pterotracheidae the larval shell is cast off after metamorphosis
- Adult shell in Atlantidae and Carinariidae bears a keel, which extends outward from the last shell whorl in atlantids and extends anteriorly from the shell in carinarids


Figure. Left: Atlanta peroni, viewed from the right side. Note the transparent shell and the keel that extends outward from the shell margin. Right: visceral mass and gills contained within the transparent shell of Carinaria galea, viewed from right side. The well-developed keel extends from the anterior edge of the shell. The stalked visceral mass is attached to the posterior part of the trunk (see the second title illustratoin). © 2005 Roger R. Seapy
- Adult shell calcareous (aragonitic) in adult Carinariidae. In Atlantidae shell and keel are either calcareous (Atlanta), composed exclusively of a cartilaginous-like material, conchiolin (Oxygyrus), or with a calcareous shell and a conchiolin keel (Protatlanta) (see Atlantidae page)

Figure. Left eye of a small (ca. 20 mm body length) Pterotrachea hippocampus in dorsal view. Also in the photograph is the left statocyst, which consists of an outer capsule and an inner, mineralized statolith (at the tip of the pointer). © 2005 Roger R. Seapy
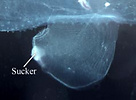
Figure. Swimming fin with posteroventral sucker in a male Carinaria japonica, viewed from the right side. © Roger R. Seapy
Comments
The three families comprising the superfamily Pterotracheoidea can be most readily distinguished by the following characters:
| Family | Adult shell | Body can retract into shell | Adult body size | Location of swimming fin |
|---|---|---|---|---|
| Atlantidae | present | yes | microscopic (shell diameter < 1 cm) | extending anteriorly beneath head |
| Carinariidae | present | no | macroscopic (adult body length > ca. 2 cm) | opposite visceral mass at posterior end of trunk |
| Pterotracheidae | absent | not applicable | macroscopic (adult body length > ca. 2 cm) | on trunk ventrum between visceral mass and head |
| License | http://creativecommons.org/licenses/by/3.0/ |
| Rights holder/Author | Roger R. Seapy, Tree of Life web project |
| Source | http://tolweb.org/Pterotracheoidea/27801 |
Heteropods are dioecious (separate sexes) and exhibit sexual dimorphism. Males have a prominent penis and penial appendage where sperm are packaged into spermatophores (see first photograph below). Sperm are transferred to the penis from the testis, located in the visceral mass, by an external ciliated sperm groove. During copulation, spermatophores are transferred to females. Unfortunately, mating behavior has only been observed in Pterotrachea hippocampus (by R. Harbison; reported in Lalli and Gilmer, 1989). Fertilized eggs are deposited in mucoid egg strings (see second photograph below) that eventually break free from the female, except in Firoloida desmarestia which has a permanently attached tubular filament that holds the developing embryos (Owre, 1964; see F. desmarestia page).
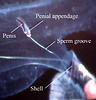

Figure. Left - Side view of posterior region of trunk of Carinaria japonica, male. Penis and penial appendage lie parallel to each other; penis contains brown spermatophore. Right: side view of visceral nucleus in Pterotrachea coronata, female. The egg string is released from the oviduct opening, which is surrounded by transparent "lips". © 2005 Roger R. Seapy
All heteropods have a free-swimming, planktotrophic veliger larva. Larvae hatch from the egg within a few days of fertilization. The veliger possesses a calcareous, dextrally-coiled (right-handed) shell and a flexible, cartilaginous operculum. The larval velum (ciliated swimming and feeding organ) is initially small and bilobed. With growth each lobe forms into two (pterotracheids) or three (atlantids and carinariids) pair of long, slender lobes (see drawing below). Following metamorphosis the larval shell is retained as the protoconch of the adult shell in atlantids and carinariids, but is cast off after metamorphosis in pterotracheids. The larval operculum is either retained (atlantids) or cast off (carinariids and pterotracheids). The duration of the larval stage is not known, nor is the age or life span of adults. The largest heteropods are the carinariids (body length >680 mm in Carinaria cristata; see C. cristata page) followed by the pterotracheids (to 330 mm in Pterotrachea coronata; Lalli and Gilmer, 1989).


Figure. Left: sketch of veliger larva of Carinaria lamarcki with six slender, ciliated velar lobes. Drawing from Thiriot-Quievrèux (1973). Right: photograph of late veliger larva of Oxygyrus keraudreni, shell diameter ca. 1.0 mm. Head, proboscis and opercular lobe extend from shell; velar lobes retracted into shell (light violet, frilly structures; left side of photograph); . © 2005 Roger R. Seapy
| License | http://creativecommons.org/licenses/by/3.0/ |
| Rights holder/Author | Roger R. Seapy, Tree of Life web project |
| Source | http://tolweb.org/Pterotracheoidea/27801 |
